Change in Entropy Formula
Where change in entropy. For any such process.
Δ S S B S A A B d q rev T A B d q irrev T.

. ΔS surr -ΔHT. The entropy formula is given as. For gases there are two possible ways to evaluate the change in entropy.
The Gibbs entropy formula is. DE dQ - dW. Since entropy is a state function the entropy change of the system for an irreversible path is the same as for a reversible path between the same two states.
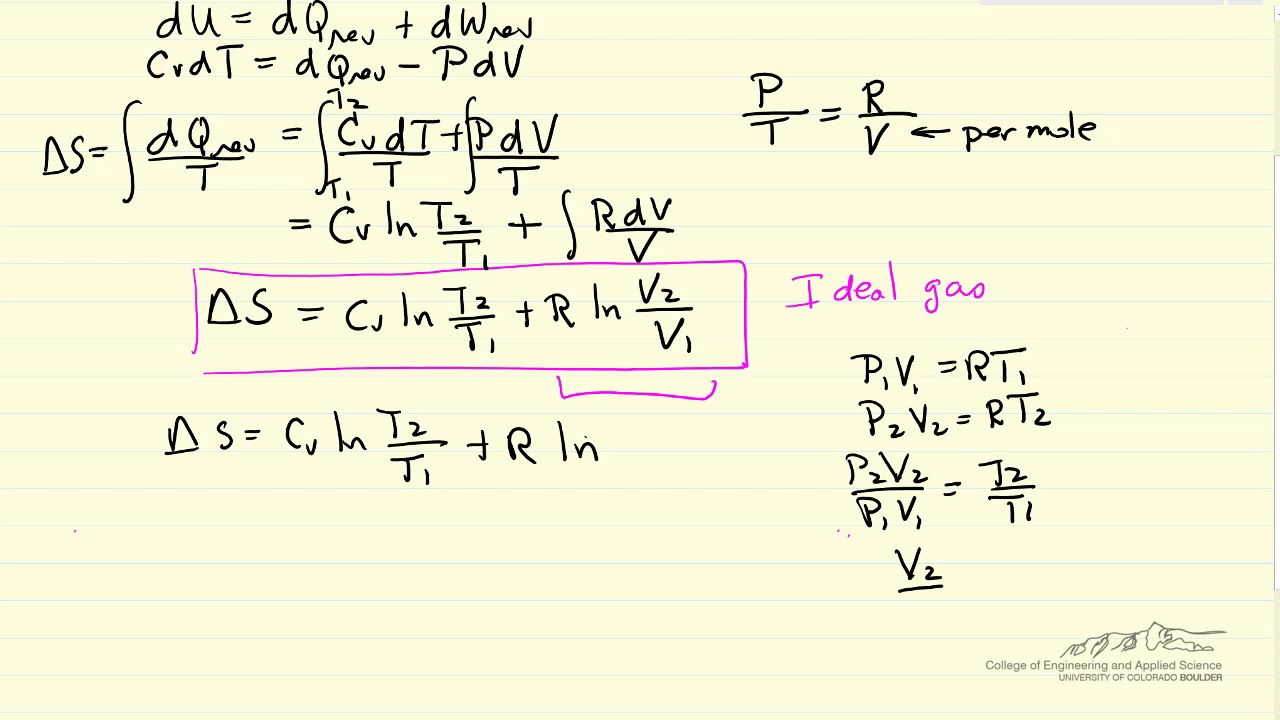
Entropy is typically considered a function of temperature and either volume or pressure. However the heat transferred to or. S q reviso T.
Entropy is a state function that is often erroneously referred to as the state of disorder of a system. The change in entropy of the surroundings after a chemical reaction at constant pressure and temperature can be expressed by the formula. Formula to calculate entropy change.
Enter the scientific value in exponent. Entropy is the measure of disorders or randomness of the particular system. When we hold temperature constant an isothermal process and change one of the.
It can also be explained as a. If the process is at a constant temperature then where ΔS is the change in entropy q rev is the reverse of the heat and T is. If the process is at a constant temperature then.
If we add the same quantity of heat at a higher temperature and lower temperature randomness will be maximum at a lower temperature. It arises directly from the Carnot cycle. Calculate the entropy of a reaction if the amount of heat transfer is 9200 joules and.
Since it depends on the initial and final state of the system the absolute value of entropy cannot be determined. Where E is the internal energy. Qualitatively entropy is simply a measure how much the energy of atoms.
Change in Entropy textDelta EntropytextEntropy in-textEntropy outtextEntropy gen Instructions to use calculator. T is the temperature in Kelvin. For any such process.
The inequality d q rev d q irrev together with the definition of entropy in terms of reversible heats gives. Entropy change what you end up with - what you. Entropy change can be.
We can calculate Entropy using many different equations. The entropy of a system. Entropy can be calculated using many different equations.
The inequality d q rev d q irrev together with the definition of entropy in terms of reversible heats gives. Δ S S B S A A B d q rev T A B d q irrev T. You ended up with 1 mole of carbon dioxide and two moles of liquid water.
The degree of disorder or randomness of a system is known as Entropy. Entropy is a fundamental function of a state. We begin by using the first law of thermodynamics.
It is the thermal energy of a system that is not available for useful work. Reverse of the heat. Total entropy at the end 214 2 699 3538 J K -1 mol -1.
Q is the heat transfer.

Thermodynamics Formula Of Entropy Physics Stack Exchange

How To Calculate Entropy Changes Liquids Solids And Phase Changes Youtube
How To Calculate Entropy Changes Ideal Gases Youtube

Entropy Change For Melting Ice Heating Water Mixtures Carnot Cycle Of Heat Engines Physics Youtube
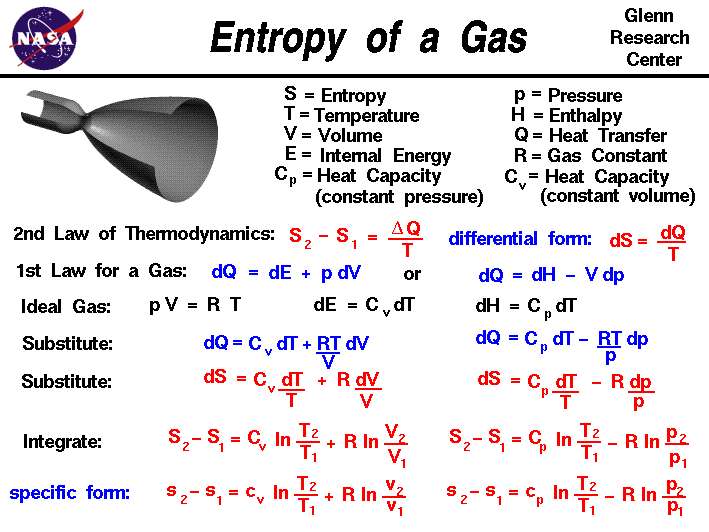
Comments
Post a Comment